Hours after the FDA and CDC recommended resuming use of the Johnson & Johnson COVID-19 vaccine, New York state government said it would follow their guidance and again make the one-dose shot available to residents.
The city also agreed on Saturday to again provide the Johnson & Johnson vaccine.
The federal agencies called for a pause on the Johnson & Johnson vaccine back on April 13 after six women who received the shot developed blood clots. The pause was out of an abundance of caution to examine the side effect; more than 6 million Americans had received the J&J vaccine up to that point.
Following further investigation, both the FDA and CDC gave the green light on April 23 for states and health care providers to again use the vaccine, but with a warning that women younger than 50 who seek to receive the shot face the rare risk of blood clots with low platelets after vaccines.
The risk of blood clots have not been seen in other vaccines used.
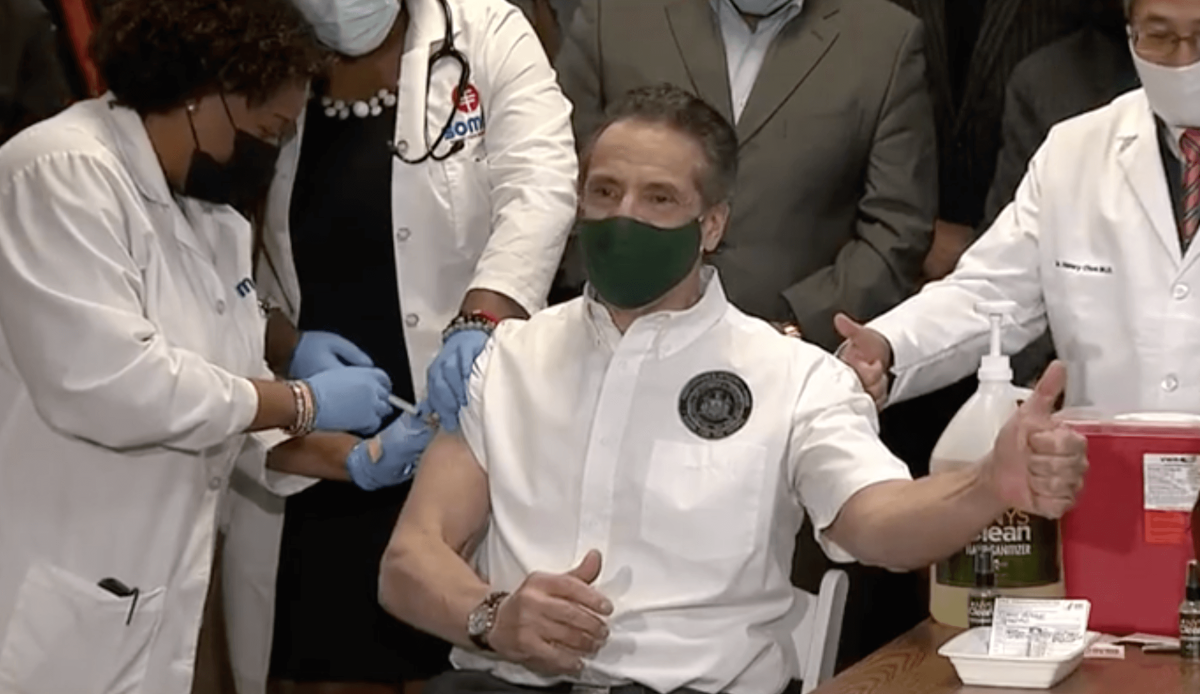
On Saturday morning, Governor Andrew Cuomo and state Health Commissioner Dr. Howard Zucker said they would allow use of the J&J vaccine in New York immediately.
“World-renowned public health experts from the federal government and our own independent state task force have reviewed the data and reaffirmed that the use of the Johnson & Johnson vaccine can resume. The state of New York will resume administration of this vaccine at all of our state-run sites effective immediately,” said Cuomo, who previously received the Johnson & Johnson vaccine. “The vaccine is the weapon that will win the war against COVID and allow everyone to resume normalcy, and we have three proven vaccines at our disposal. I urge every New Yorker to take whichever one is available to them first. The sooner we all get vaccinated, the sooner we can put the long COVID nightmare behind us once and for all.”
“The data has shown the vaccine’s known benefits far outweigh the potential and extremely rare risks, but we urge anyone with questions about the COVID-19 vaccines to speak with their healthcare provider. We will continue to communicate regular updates and guidance from the federal government to providers and the general public about the Johnson & Johnson vaccine and all vaccines on the market,” Zucker added. “We encourage all New Yorkers to get whichever vaccine is available to them, as quickly as possible, so we can finally defeat this virus and continue our path towards fully reopening our communities and economy.”
Thereafter, Mayor Bill de Blasio and city Health Commissioner Dr. Dave Chokshi — both of whom also received the J&J vaccine earlier this spring — also said the city would make the vaccine available to New Yorkers immediately.
“The one-shot Johnson & Johnson vaccine is back and New York City will immediately resume our innovative mobile, pop-up, and homebound vaccination programs with this shot,” said de Blasio. “I received the Johnson & Johnson vaccine along with our Health Commissioner Dr. Chokshi. We know firsthand that the vaccine is safe and effective, and now it’s easier than ever to get your shot.”
“The joint CDC and FDA recommendation to resume the use of the Johnson & Johnson vaccine with an updated warning shows that our safety system works,” Chokshi added. “I received the Johnson & Johnson vaccine, and I trust it is safe and effective. If I had the chance to decide again, knowing what I know now, I would still choose to receive the Johnson & Johnson vaccine.”
The Governor’s office reported Friday that more than 14 million COVID-19 vaccine doses have been administered in New York state to date, including 184,119 through the state’s vaccine distribution network over the previous 24 hours. Nearly half of all New Yorkers (43.2%) have received at least one COVID-19 vaccine dose.
While the Johnson & Johnson vaccine is a one-dose remedy, most New Yorkers have received the two-shot Pfizer or Moderna vaccines. During the J&J pause, New Yorkers who had made appointments to receive the vaccine were instead provided with the Pfizer shot.
Vaccine differences
Unlike the Johnson & Johnson vaccine — which, like traditional vaccines, introduce a weakened version of a virus into the body to boost an immune response — the Pfizer and Moderna shots are mRNA-based vaccines which involve the use of weakened copies of the COVID-19 protein in order to train the body to fight the virus. These vaccines do not alter the body’s genetics.
The CDC says that the Johnson & Johnson vaccine has been 66.3% effective in clinical trials at preventing COVID-19 illness, and has high efficacy in preventing hospitalization and death among those who did contract the virus.
According to the CDC, the Pfizer vaccine has been “100% effective” in preventing severe COVID-19 disease. An FDA study found the efficacy rate to be 95.3%. The Moderna vaccine efficacy rate, meanwhile, was 94.3%.
The most common side effects of the Pfizer and Moderna vaccines have been brief periods of fever, fatigue, aches and pain in the injection site, usually within 24 to 48 hours after the dose is administered.